Abstract
Introduction
The treatment of acute myeloid leukemia (AML) has evolved tremendously. Recently, venetoclax with hypomethylating agents (HMA/ven) demonstrated durable responses in the frontline and relapsed/refractory (R/R) settings. This regimen is now standard of care for older adults or those unfit for intensive induction chemotherapy (DiNardo CD, N Engl J Med, 2020). Our institution also often uses HMA/ven to treat fit patients (pts) with high risk disease characteristics. Because HMA/ven was studied in transplant-ineligible pts, outcomes following potentially curative allogeneic hematopoietic stem cell transplantation (HSCT) remain unknown. This retrospective study aims to describe characteristics and outcomes of pts treated with HMA/ven who proceeded to HSCT.
Methods
Adult pts diagnosed with AML and treated with HMA/ven either in the frontline or R/R setting between 1/2010 and 2/2020 at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University were identified. Hypomethylating agents included either azacitadine or decitabine. Data were collected and analyzed based on demographics, laboratory and clinical characteristics, and disease and toxicity outcomes. Efficacy endpoints included complete remission (CR), CR with incomplete hematologic recovery (CRi), and CR with incomplete platelet recovery (CRp). Survival curves for overall survival (OS) and leukemia-free survival (LFS) were calculated using the Kaplan-Meier method. Univariate analyses were performed to determine impact of clinical variables on outcomes (significance defined as p≤0.05). Cohorts were compared using χ 2 or Fisher's exact test for categorical variables and the unpaired t-test for continuous variables.
Results
Clinical and demographic features at time of diagnosis are listed in Table 1. In total, 257 pts received HMA/ven. Of these, 36 pts received a HSCT, which was the population analyzed in this study. In the front-line setting 11 (31%) pts received HMA/ven and 25 (69%) pts received HMA/ven for R/R disease. 25 (69%) pts received azacitadine and 11 (31%) pts received decitabine (5 days, n=5, 14%; 10 days, n=6, 17%). Based on ELN guidelines, 23 (64%) pts had adverse risk disease at diagnosis. Response to HMA/ven in the pre-transplant setting is shown in Table 2. Of 35 evaluable pts, 34 achieved remission (CR, n=32, 91%; CRi, n=1, 3%; CRp, n=1, 3%).
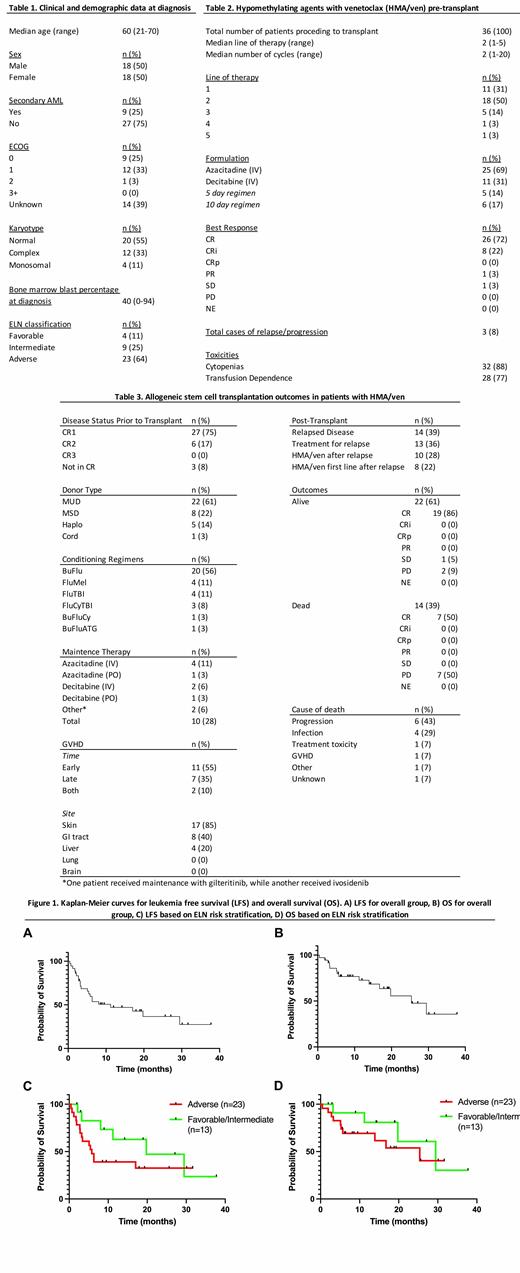
Table 3 shows outcomes following HSCT. 14 (39%) pts relapsed post HSCT and 13 (36%) pts received treatment for relapse. With a median follow-up of 11.6 months, median LFS from time of transplantation was 11.2 months. Median OS was not reached over follow up period but estimated to be 25.4 months. There was a significant difference in rates of relapse based on ELN classification at diagnosis (p=0.0296). In comparison, presence of complex/monosomal karyotypes (p=0.593), blast percentage at diagnosis (p=0.456), donor type (p=0.484), and number of previous lines of therapy (p=0.822) did not predict for relapse. Median LFS in adverse and favorable/intermediate risk ELN groups was 5.8 and 19.8 months, respectively. Median OS in adverse and favorable/intermediate risk ELN groups was 25.4 and 29.5 months, respectively.
Following transplant, 10 (28%) pts received maintenance therapy with a median of 5 cycles (range 1-14); 8 pts (22%) received HMA/ven maintenance following HSCT. There was no significant difference in relapse rates between those who received maintenance therapy (n=6, 43%) and those who did not (n=8, 57%) (p = 0.107). Median time to relapse from HSCT was 4.42 months in those who received maintenance therapy compared to 2.98 months in those who did not receive maintenance therapy (p=0.370).
Following relapse, 10 (28%) pts were retreated with HMA/ven, but less than half (n=4, 40%) had a response. To date, 22 (61%) pts are alive with the majority (n=19, 86%) in remission. 14 (39%) pts died with half in remission at the time of death.
Conclusions
Our study showed that HMA/ven can feasibly be used not only to bridge to transplant, but to achieve durable remissions post HSCT. For those pts that relapsed post HSCT, duration of remission was very short. ELN classification was the only factor that informed relapse risk. Prospective studies must be done to understand which cytogenetic and molecular subgroups benefit the most from HMA/ven prior to transplant.
Abaza: BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Altman: Biosight: Consultancy, Other: Travel fees, Research Funding; Fujifilm: Research Funding; Kura: Research Funding; Immunogen: Research Funding; Kartos: Research Funding; Daiichi Sankyo: Consultancy; ALZ Oncology: Research Funding; Theradex: Consultancy, Other: Advisory boards; Syros: Consultancy; Amgen: Research Funding; Aprea: Research Funding; Boehringer Ingelheim: Research Funding; Astellas: Consultancy, Other: Advisory Board, Research Funding; GlycoMimetics: Other: Participation on an advisory board; AbbVie: Consultancy, Other: Advisory Board, Research Funding; BMS: Research Funding; Kura Oncology: Consultancy. Dinner: Pfizer: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal